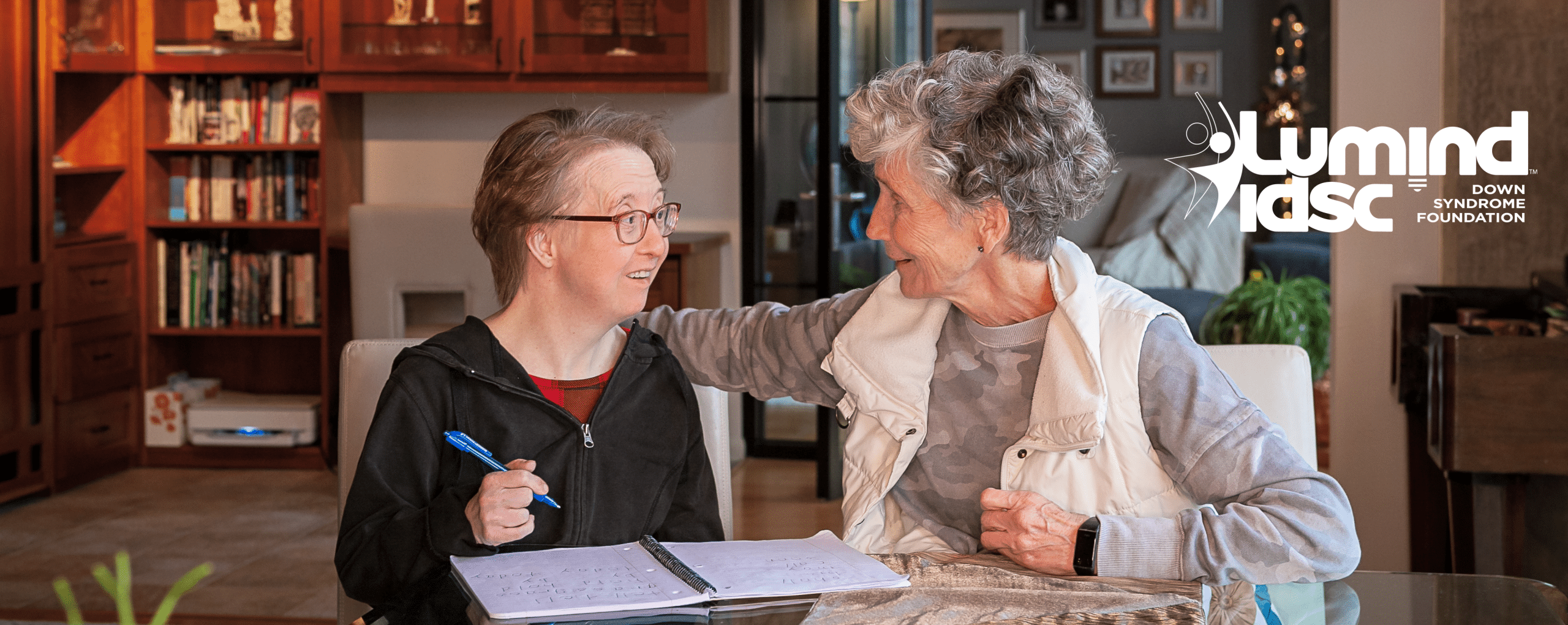The Down syndrome community demands equal access to clinical trials, drug therapies, and diagnostics that could change our lives.
I believe that people with Down syndrome deserve equal access to, and insurance coverage for, life-changing drugs and critical diagnostic tests.
The entrenched policies we change today will improve healthcare infrastructure and make access to treatment more equitable for people with Down syndrome in the long term.
In signing this petition, I add my name to the thousands of people who care about the health and well-being of people with Down syndrome.
Today, our fight is focused on urgent inequities around new Alzheimer’s disease therapies and diagnostic tests.
In bringing attention to the issue of Down syndrome equity now, our community is paving the way for access to future breakthrough treatments for other diseases and conditions. The entrenched policies we change today will improve healthcare infrastructure and make diagnoses and access to treatments more equitable for people with Down syndrome in the long term.